| Complexity level: | 7 |
| Project cost ($): | 20 |
| Time required: | 1 day to prepare, 3 days for observation |
| Material availability: | Easily found |
| Safety concerns: | Not applicable |
Hypothesis
Lower air pressure in the surroundings will cause helium gas to escape at a faster rate, from a balloon.
Overview
Diffusion - is the transfer of molecules from an area of high molecule density to an area of lowerdensity. In this experiment diffusion occurs when the helium gas molecules inside the balloon (are of high molecule density) move out into the airspace surrounding the balloon. The air pressure of the room in which the experiment is conducted, is varied by repeating the experiment on different levels of a tall building.
Scientific Terms
Materials
The materials required for the experiment:
- 60 balloons
- 1 tank of helium gas
- A 500mm ruler and 2 wooden blocks
- 60 nails to hold down the balloons
- 100m long string to tie the balloons
Procedure
- For this experiment, the independent variable is the room air pressure. The dependent variable is the outer diameter of the balloon, which reduces with time. This is determined by using a ruler and 2 wooden blocks to measure the diameter of the balloons. The constants (control variables) are the temperature in the room, the humidity level in the room and the type of gas used to inflate the balloons.
- The experiment is carried out in an air-conditioned room to maintain a constant temperature and humidity. It is performed on the ground floor, 30th storey and 60th storey of a tall building, in order to provide environments with different surrounding air pressure.
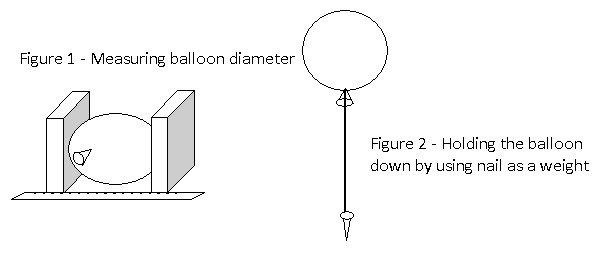
- The experiment is started on the ground floor. 20 balloons are inflated using helium gas up to a diameter of 300mm. Figure 1 shows how the diameter of each balloon is measured. The balloons are tied to a nail using a string as shown in figure 2. The nail acts as a weight to keep the balloon in place
- The diameter of the balloon is measured once every 2 hours to determine the rate at which the helium gas escapes from the balloons. The results are recorded in the table below.
- Procedures 3 and 4 are again repeated on the 30th floor and 60th floor of the building. The results are recorded in the same table below.
Results
You should observe that the balloons on the 60th floor will reduce in size and diameter, at a faster rate than the balloons on the ground floor. In other words, the helium gas will escape more quickly from the balloons located on the highest floor.
Use the table below to help record all of your measurements.
| Balloon no: | Ground floor (diameter in cm) | 30th Floor (diameter in cm) | 60th Floor (diameter in cm) | ||||||||||||
| Start | 2hr | 4hr | 6hr | 8hr | Start | 2hr | 4hr | 6hr | 8hr | Start | 2hr | 4hr | 6hr | 8hr | |
| 1 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.5 | 23.5 | 20.5 | 18.5 |
| 2 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.5 | 25.5 | 24.5 | 24.0 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 3 | 30.0 | 28.5 | 27.5 | 26.5. | 25.5 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 4 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 5 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 26.5 | 24.5 | 23.5 | 23.0 | 30.0 | 25.5 | 22.5 | 19.5 | 17.5 |
| 6 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 7 | 30.0 | 27.5 | 26.5 | 25.5. | 24.5 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 8 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 9 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.5 | 25.5 | 24.5 | 24.0 | 30.0 | 26.5 | 23.5 | 20.5 | 18.5 |
| 10 | 30.0 | 28.5 | 27.5 | 26.5. | 25.5 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 11 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 26.5 | 24.5 | 23.5 | 23.0 | 30.0 | 25.5 | 22.5 | 19.5 | 17.5 |
| 12 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 13 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.5 | 23.5 | 20.5 | 18.5 |
| 14 | 30.0 | 27.5 | 26.5 | 25.5 | 24.5 | 30.0 | 27.5 | 25.5 | 24.5 | 24.0 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 15 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 16 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 26.5 | 24.5 | 23.5 | 23.0 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 17 | 30.0 | 28.5 | 27.5 | 26.5. | 25.5 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 25.5 | 22.5 | 19.5 | 17.5 |
| 18 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 19 | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| 20 | 30.0 | 27.5 | 26.5 | 25.5. | 24.5 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
| Average | 30.0 | 28.0 | 27.0 | 26.0. | 25.0 | 30.0 | 27.0 | 25.0 | 24.0 | 23.5 | 30.0 | 26.0 | 23.0 | 20.0 | 18.0 |
Use the graph below to plot the results of above observation. 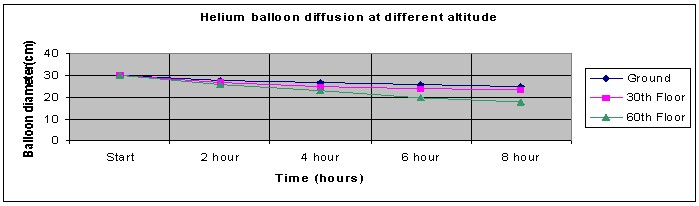
Conclusion
The hypothesis is proven to be true. Higher floors have a lower atmospheric pressure and therefore lower air density. This causes the helium gas which is at a higher density to escape more rapidly due to diffusion. The helium gas is able to escape through microscopic holes that naturally exist in the walls of latex balloons. These small holes are larger than helium atoms. It is because of diffusion, that balloons grow smaller over time.
These helium balloons are commonly used in parties and are extremely popular with children. In more advanced applications, they are also used to gather data for weather forecasting.
Also consider
What would happen if the experiment was conducted using other types if gases?
How will different room temperatures affect your results? What happens if you vary the humidity levels of the surrounding air?
References
- Diffusion - http://en.wikipedia.org/wiki/Diffusion
- What causes helium to escape from balloons ?http://www.helium.com/items/1246954-what-causes-helium-to -escape-from-balloons

