| Complexity level: | 5 |
| Project cost ($): | 80 |
| Time required: | 1 day to prepare, 4 weeks for observation |
| Material availability: | Easily found |
| Safety concerns: | Not applicable |
Hypothesis
Using polymer sealants will help a car paint last longer by providing a protective layer over the paint, preventing damage by various substances such as water and acid rain.
Overview
Corrosion
Corrosion is actually the process by which the surface of a substance (such as metal) undergoes a chemical reaction with elements in its surrounding. One example, is the process of oxidation - this occurs when metal is placed in the presence of water and oxygen. This is how rust forms. Painting is one of the most common methods used to prevent rust from occurring. However paint itself is vulnerable to damage by industrial and naturally-produced chemicals and therefore additional coatings such as wax or polymer sealants are applied over the paint to protect it. This prevents the paintwork itself from corroding.
Scientific Terms
Materials
The materials required for the experiment:
- 20 pieces of metal from the exterior body of a car ?cut into sheets of 20cm by 20cm (these metal sheets can be purchased from car work shops)
- 1 large can of automobile bodywork spray paint
- Polymer sealant (example Zaino Brothers Z5)
- 2 eggs
- 1 bottle of Clorox cleaner
- Bird dropping (fresh)
- 1 bottle of soy sauce
- 1 bottle of tap water
- 1 bottle of sugar diluted in water
- 1 bottle of mouthwash
- 1 bottle of salad cream
- 1 bottle of cooking oil
- 1 can of shaving cream
Procedure
- For this experiment, the independent variable is the use of protective sealant and the type of materials used to act as the corrosive agent. The dependent variable is the appearance of rust or corrosion on the metal sheets over time. The constants (control variables) are the type of metal sheets used, the temperature, amount of sunlight and type of paint used.
- The experiment is begun by spray painting all of the 20 metal sheets and allowing them to dry for 8 hours.
- 10 plates are separated and applied with a coating of protective sealant. The metal sheeets with the protective sealants are marked (i.e. rotective sealant?written with permanent marker in one corner) and numbered 1 to 10.
- The following 10 types of materials are spread on the metal sheets with and without the protective sealant ?egg, Clorox, bird dropping, soy sauce, tap water, sugar water, mouthwash, salad cream, cooking oil and shaving cream.
- The metal plates are kept in a place where they will not be disturbed, and where the amount of sunlight and heat is constant (eg. air conditioned room). They are observed closely for appearance of corrosion and the results are recorded in the table below once a week, for four weeks.

Results
You should observe that some of the materials used to induce corrosion were very effective in doing so even in the presence of the polymer sealant. Eggs, Clorox and shaving cream are very harmful to paintwork.
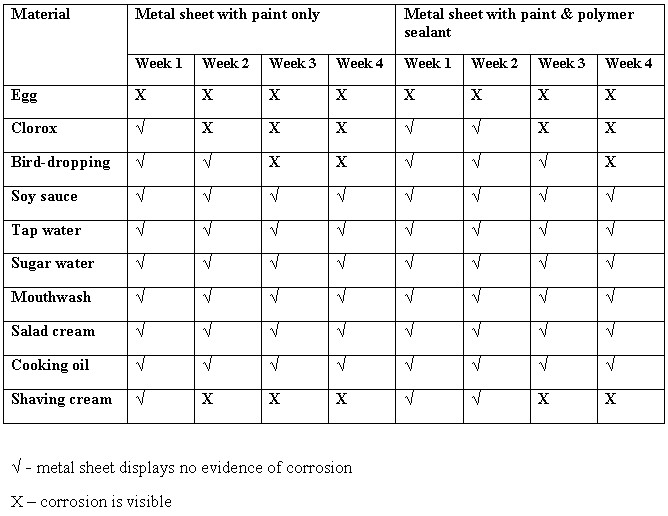
Conclusion
The hypothesis is proven to be true.
The paintwork on the metal sheets without the polymer coating began to corrode sooner than those which had coating. Use of polymer sealants on car surfaces do not prevent rust from occurring but only delays the process of corrosion. The protective coating acts as a first line of defense against corrosion-inducing chemicals, by allowing the protective coating to be damaged first before the corrosion is able to reach the surface of the paint.
The protective coating industry for automobile and shipping is very big business. A lot of money is spent in research and development to create new and improved products that provide much better protection under different environments
Also consider
What would happen if the experiment was performed using other brands of paint.
What if other metals such as zinc or aluminium were used?
How will different types of protective coatings, such as wax affect the results?
What if the metal sheets were exposed to intense ultra violet rays? Or what would be the effect of increasing the room temperature?
References
- http://en.wikipedia.org/wiki/Corrosion

