| Complexity level: | 5 |
| Project cost ($): | 10 |
| Time required: | 1 hour to prepare, 10 minutes for observation. |
| Material availability: | All materials mentioned should be available from your school chemistry laboratory. |
| Safety concerns: | Students should be careful as they are dealing with electricity and chemical reactions in this experiment. Adult supervision required at all times. |
Hypothesis
Zinc and copper wet cell batteries produce the highest voltage.
Overview
What are electrodes and electrolytes?
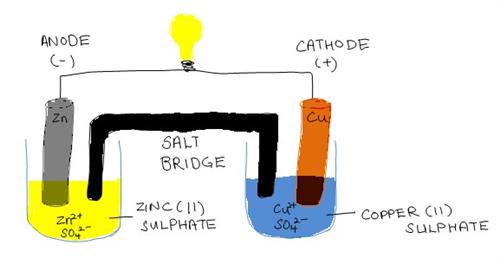
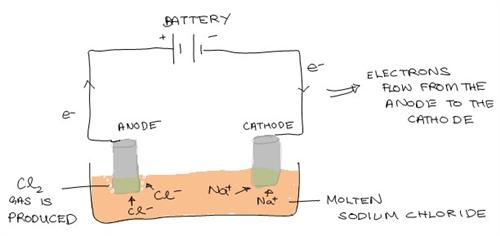
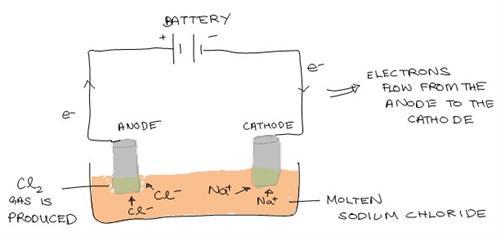
An electrode is an electrical conductor connected to a nonmetallic part of a circuit. Electrodes are referred as either the anode or cathode in an electrochemical cell. The anode is the electrode where current leaves the cell and oxidation happens. On the other hand the cathode is where current enters the cell and reduction happens.
An electrolyte is an aqueous type of substance that contains free moving ions. It is the presence of these free moving ions that makes the substance electrically conductive.
Types of electrochemical cells
There are two types of electrochemical cells, galvanic (voltaic) cell and electrolytic cell.
Both cells contain electrodes on which oxidation and reduction happens. However, the cathode and anode are charged differently. In the galvanic (voltaic) cell, the anode is negatively charged and the cathode is positively. It is vice-versa for an electrolytic cell. Here, the anode is positively charged and the cathode is negatively charged.
As such, due to the nature of the anode being negatively charged in the galvanic (voltaic) cell, it provides a ready source of electrons, which are negatively charged. As such, there is spontaneous reaction. In contrast, activity is non spontaneous in an electrolytic cell and in order for the electrolysis reaction to take place, electrical energy is required.
Due to this reason, galvanic (voltaic) cells are commonly used in batteries
However, it is common in both cells, that the anode is the electrode where oxidization occurs and the cathode is where reduction occurs.
A Galvanic (Voltaic) Cell
An Electrolytic Cell
Scientific Terms
Materials
The materials required for this science project are:
- Zinc sheet
- Copper sheet
- Lead sheet
- 200ml Zinc nitrate solution
- 200ml Copper nitrate solution
- 200ml Lead nitrate solution
- Porous cup
- 2 beakers
- 1 voltmeter
- Gloves
- Plastic safety goggles
- A piece of Sandpaper
Procedure
1. The independent variable of this experiment is the type of electrode used. The dependent variable is the voltage. The constants of this experiment are the following: the amount of electrolyte used, the length of wires and the size of copper sheets used.
2. Take necessary safety steps by putting on goggles and gloves.
3. Rub the copper, zinc and lead sheets against the sandpaper to remove any deposits.
4. Pour 100ml of zinc nitrate solution into a beaker. Put the zinc sheet into the solution.
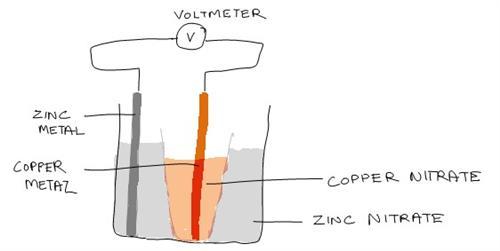
5. Pour about 50ml of copper nitrate solution into the porous cup and immerse the copper sheet into the solution.
6. Cut the wire into 2 pieces of about 10cm each, connect one wire to the copper sheet and the other to the zinc sheet. After that, connect the free ends to the voltmeter.
7. If the voltmeter shows a negative reading, switch the ends of the wire.
8. Later, place the porous cup into the beaker and read the voltmeter. Record your reading in a table.
Illustration of the Experiment Set Up
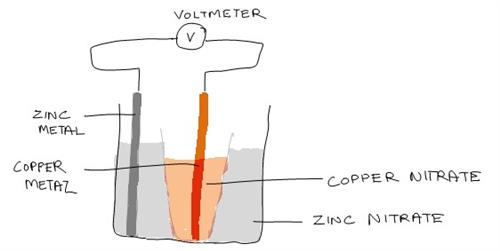
9. Repeat 5 trials.
10. Next, prepare the lead half-cell in another beaker by pouring 100ml of lead nitrate solution into the other unused beaker. Remember to rub the copper strip against the sandpaper to ensure that the metal is exposed to the solution. Rinse the porous cup with distilled water and immerse it into the lead nitrate solution.
11. Repeat the above steps for the zinc half-cell.
Results
The results obtained in this experiment are recorded in the table below.
| Type of electrochemical cell | Voltage (mV) |
| Zinc-copper | 100 |
| Lead-copper | 501 |
| Lead-zinc | 489 |
*The above readings are the average obtained after 5 trials.
For the zinc-copper cell, 100 millivolts of electricity is produced.
For the lead-copper cell, 501 millivolts of electricity is produced.
For the lead-zinc cell, 489 millivolts of electricity is produced.
Conclusion
The results obtained from the experiment suggest that the hypothesis should be rejected. According to this experiment, lead-copper cell produces the greatest amount of electricity.
This experiment provides information about which type of material is most suitable as to be used as electrodes in batteries. However, companies should take into consideration for problems such as cost-effectiveness, material availability, safety and environmental effect before mass producing a certain product.
Also consider
What would happen if more electrolyte solution was added?
Would increasing the temperature of the solution affect the voltage produced?
What steps should you take to minimize errors in your experiment?
What other materials can you use for the electrolytes and electrodes?
References
http://www.obbligato.com/productsSite/software/F01/cell-types.pdf

