Hypothesis
The empty soda can having a lower internal air pressure, will collapse inwards due to the higher water pressure outside the can.
Overview
Air pressure
Air molecules have a certain mass and when these molecules collide with a surface, pressure is felt on that surface. The molecules in the air contain kinetic energy and are always moving. The moving molecules are always colliding with one another or onto other surfaces creating the phenomenon that we call pressure.
Let us say we have a sealed container containing air. If we increase the amount of air molecules in the container, the number of air molecules colliding with the inner walls of the container will increase and so will the air pressure. If the number of air molecules inside the container is reduced, the air molecules colliding with the inner walls of the container will also reduce along with the air pressure inside the container.
Another method of varying the air pressure inside the container is temperature. Increasing the temperature of the container will cause the molecules inside the container to have a greater amount of kinetic energy. This will make the molecules collide more frequently amongst themselves and on the inner surface of the container causing the air pressure inside to be greater. Cooling down the container will reduce the kinetic energy and also lessen the collisions within the inner walls of the container, causing the air pressure to fall.
Scientific Terms
Molecules, kinetic energy, air pressure
Conclusion
The hypothesis that an empty soda can having a lower internal pressure will collapse inwards due to the higher exterior water pressure is proven to be true.
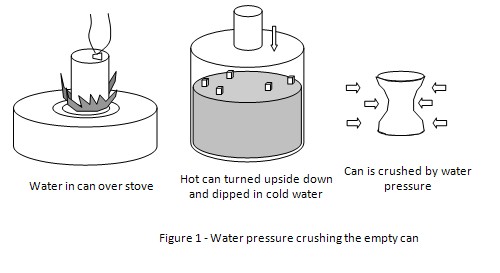
When the water in the soda can starts to boil, the air is pushed out (displaced) by the steam from the boiling water. The air space in the can is replaced by the steam. When the can is turned upside down and pushed into the cold water, the steam quickly cools and turns back into water droplets in an instant, creating a vacuum inside the can. The water pressure outside the can immediately starts to push against the walls of the soda can. This causes the walls of the soda can to collapse inwards.
Also consider
Try to repeat the experiment with different types of metal containers.
Try another experiment by capping an empty plastic bottle tightly and placing it in the freezer.
References
Atmospheric pressure - http://en.wikipedia.org/wiki/Atmospheric_pressure
Air pressure -http://www.uwsp.edu/geo/faculty/ritter/geog101/textbook/circulation/air_pressure_p_1.html
Related videos
Hey there! Here are some awesome videos about this science project that we think you'll really like. They're not only super fun, but they'll also help you learn more about the science behind the project. So sit back, relax, and get ready to have some fun!!