Purpose
The purpose of this experiment was to determine if the level
of turbidity in water affected the amount of dissolved oxygen.
I became interested in this idea because I am interested in water quality
and environmental issues. I am interested in water quality because
dissolved oxygen keeps fish and other animals alive.
The information gained from this experiment could be useful to the fish
and wildlife foundation and dam operators.
Top of page
Hypothesis
My hypothesis is that the more turbidity levels in water increase, the
more dissolved oxygen levels decrease. I base my hypothesis on information
that I have found in numerous sources, including books, encyclopedias,
and Internet Sites.
Top of page
Experimental Design
The constants in this study were:
~ Same amount of water tested
~ Same testing procedures
~ Test the same time each day
~ Same chemicals used
The manipulated variable was the level of turbidity in water.
There were three different locations tested. Each different location
had three different levels of turbidity.
The responding variable was the amount of dissolved oxygen in different
levels of turbidity.
To measure the responding variable a dissolved oxygen kit and a turbidometer
were used.
Top of page
Materials
1 |
turbidometer |
| 2 |
bottle with stopper |
| 1 |
dissolved oxygen pillow #1 |
| 1 |
dissolved oxygen pillow #2 |
| 1 |
dissolved oxygen pillow #3 |
| 1 |
bottle of sodium thiosulfate |
| 1 |
pair of protective gloves |
| 1 |
nail clippers |
| 1 |
pair of boots/hipwaiters |
| 1 |
testing tube |
| 10 |
quart Jars |
Top of page
Procedures
1. Find an area of water that looks like it would be good to test turbidity
on.
2. Put one of the quart jars beneath the river surface and allow water
to go in.
3. Put the lid on the jar.
4. Next, grab the bottle with stopper and put it in the exact spot
that you got the water in the jar from.
5. Remove the stopper from the bottle and allow water to flow over
for two to three minutes to eliminate air bubbles.
6. Put on goggles and safety gloves.
7. Add the contents of pillow #1 and pillow #2 into the dissolved oxygen
bottle and swirl.
8. Put the stopper into the bottle. Make sure that no air gets
trapped inside. Be sure to shake well to fully mix.
9. If a brownish-orange precipitate forms oxygen is present.
IF no brownish-orange precipitate shows up then redo the sample.
10. Add dissolved oxygen pillow #3 to the sample and swirl. The
precipitate will dissolve and the water will turn yellow.
11. Pour sample into top of measuring tube, and then pour the contents
of the measuring tube into the square-mixing bottle.
12. Add one drop of thiosulfate to the square mixing bottle and swirl.
Be sure to hold the eyedropper straight over the bottle.
13. While swirling, continue to add more drops of thiosulfate into
the bottle. Count how many drops before the yellow color turns clear.
Each drop of thiosulfate equals 0.5 mg/L of dissolved oxygen.
14. Find somewhere that has a turbidometer and make an appointment
to use it.
15. Get your sample (the water in the quart jars) and take it to the
place where the turbidometer is located.
16. Get a water vial and pour the sample into it.
17. Dry the outside of the vial.
18. Put the vial into the turbidimeter and light will refract through
to the sensor to give you a turbidity reading.
Top of page
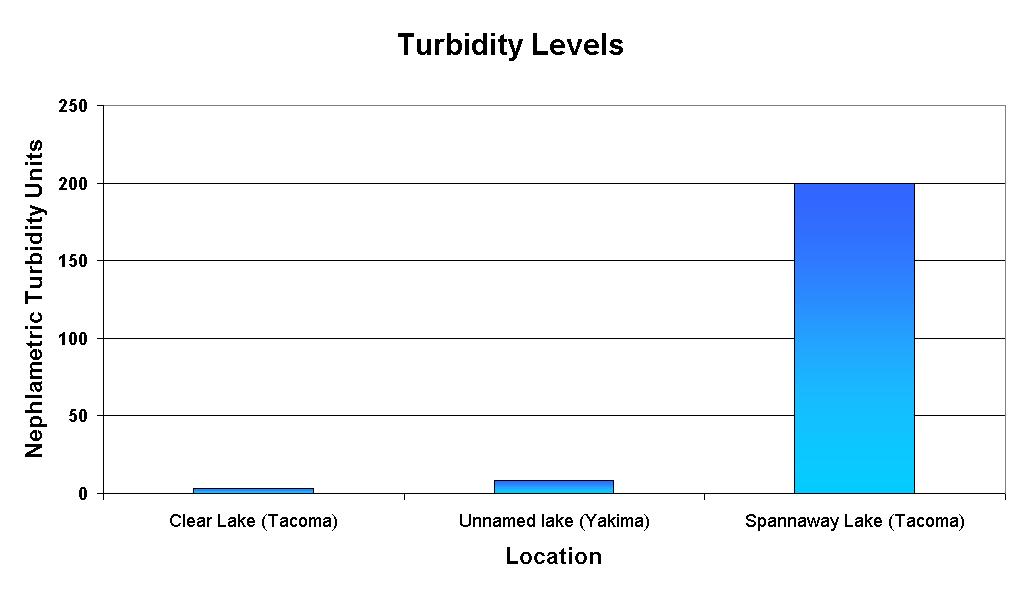
Results
The original purpose of this experiment was to determine if the level
of turbidity in water increased or decreased dissolved oxygen levels.
The results were that the test groups with the greater level of turbidity
also had the greatest level of dissolved oxygen. The groups with
the lower level of turbidity had a low level of dissolved oxygen.
Top of page
Conclusion
My hypothesis was that the more turbidity levels increase, the more
dissolved oxygen levels decrease. The results of this experiment
indicate that my hypothesis should be rejected because in fact, higher
turbidity levels actually increase the level of dissolved oxygen in water.
Because of this experiment, I wonder if turbidity affects photosynthesis?
If I were to conduct this experiment again I would have taken more samples.
This would have been more informative.
Top of page
|
Research
Report
Does Dissolved Oxygen increase or decrease with
high turbidity levels?? Do the words Turbidity and Dissolved oxygen
seem confusing? Hopefully, in reading this report, these terms
will become understandable and better enable understanding towards this
project.
This project is about the relationship
between turbidity and dissolved oxygen. The reason that this project
is important is because fish and wildlife need oxygen to live. Another
reason this project is important is that dam operator and wildlife preservers
could use the information contained in this report to know if fish and
wildlife are safe in turbid waters. They would also know, that if
turbidity levels are healthy and increase dissolved oxygen levels, they
would have to add turbidity to zoo water so that fish would have more oxygen.
Water is one of the most important recourses
on earth. Without water most things wont survive. An example
would be dehydration causes plants, animals, and people to die. Water
is the only natural substance on earth that occurs in all of the states
in matter; liquid, solid, and gas. Water is formed from compound
H2O, which means two hydrogen atoms and one oxygen atom.
Oxygen is another very important recourse
for humans to have. Without there would be no air to breathe and
mankind would die. Almost everything needs oxygen to live because
oxygen combines with other chemicals in cells to produce energy.
When oxygen combines with hydrogen it produces the liquid state of water.
Most dissolved oxygen comes from the atmosphere.
Dissolved oxygen levels change from day to day as the currents in water
change because the currents act to mix the atmospheric oxygen with water.
Turbidity is the effect of residue from
moving solids and is used as a measurement of the clarity of water.
The higher level of turbidity, the dirtier or murkier the water.
Turbidity levels usually increase as it rains because pollutants and other
matter get into the water.
There is a relationship between turbidity
and dissolved oxygen. The level of turbidity does affect dissolved
oxygen levels. The main cause for this experiment is to determine
if turbidity helps make dissolved oxygen or not. This factor
is important because then it will be know if wildlife will be safe in high
turbidity levels.
Top of page
Bibliography
"Algae," Microsoft Encarta 98 Encyclopedia, 1993
Knap, Brian, Oxygen, Danbury, Connecticut, Grolier
Educational, 1996
Mithchelle "et al," Field Manual for Water Quality
Monitoring, Dexter, Michigan, Thomson-shore, Inc., 1996
"Oxygen," Microsofn Encarta 98 Encyclopedia,
1993
Rickard, Gram, Water Energy, Milwaukee, Gareth
Stevens Childrens books, 1991.
"Solutions," Encarta, 1996
"Solvent," Encarta, 1996
"Turbidity," [online] Available Http://k12science.ati.stevens-tech.edu/curriculum/water97/turbid.html,
Tuesday, November 9, 1999
"Water," Encarta, 1998
"Water Sheds, Turbidity," [online] available
http://h2osparc.wq.ncsu.edu/info/turbid.html,
Tuesday, November 9, 19999
"Water, the Planet Earth," World Book encyclopedia
of Science, 1990
|
Top of page
Menu of 1999-2000 Science Projects
Back to the Selah Homepage
|
