| Complexity level: | 7 |
| Project cost ($): | 50 |
| Time required: | 1 day for preparation, 5 days for observation |
| Material availability: | Contact a supplier of commercial marble sealant in your vicinity |
| Safety concerns: | Warning - An adult is required for the purposes of handling oxalic acid and gloves should be worn at all times. Oxalic acid can be absorbed through the skin and it will react with calcium in the bloodstream, forming kidney stones. |
Hypothesis
Acid rain and marble surfaces
Acid rain is caused by the reaction of water vapor in the atmosphere with pollutant gasses such as nitrogen and sulfur, which make rainwater acidic. This acidic rain causes a lot of damage not only to the ecosystem and our environment, but also to buildings and sculptures that are made from limestone and marble.
Buildings and sculptures that are made from marble or limestone are particularly vulnerable to the erosion and decay caused by acid rain. These materials contain calcite, which dissolves easily in acidic water.
Oxalic acid reacts with the calcium carbonate found in marble, to form calcium oxalate on the marble's surface. Applying this acidic paste on the marble surface by spraying or rubbing will give the marble surface a brilliant shine, as well as protect it from the harmful effects of acidic rain. The film of calcium oxalate that forms on the surface is very resistant to acid rain and can therefore be used to protect wall paintings and sculptures.
Overview
http://worldacidrainsecrets.blogspot.com/2010/01/how-to-affect-acid-rain-in-buildings.html
http://pubs.usgs.gov/gip/acidrain/5.html
http://www.ucl.ac.uk/archaeology/conservation/jcms/issue4/cezar.html
Scientific Terms
Materials
The materials required for the science fair project:
- 3 pieces of marble tiles, each weighing 100 grams
- 1 bottle of oxalic acid
- 1 bottle of commercial marble sealant
- 3 beakers
- 1200 milliliters of vinegar
- 1 measuring cylinder
- 1 piece of cloth
- 1 sheet of sandpaper
- 1 digital weighing scale
Procedure
1. For this science fair project, the independent variable is the type of coating on the marble tile – one tile will not have any coating, one will be coated with an industrial sealant, and the last, with calcium oxalate. The dependent variable is the weight of the marble tiles in acid solution over time, and will be measured using a digital weighing scale. The constants (control variables) are the acidity of the vinegar solution, the amount of vinegar used, the initial weight of the marble tile and the length of time the science fair project is conducted for.
2. Clean all 3 marble tiles. Using the digital weighing scale, ensure that they weigh exactly 100g each. If a tile weighs more than 100g, reduce its weight by sanding it down with the sandpaper. Record the starting weight of the sample tiles in a table, as shown below.
3. Prepare the tiles for the science fair project. Place the first tile in a beaker labeled ‘No protection’. Polish the second tile with the commercial marble sealant according to the instructions on the packaging, and place it in a beaker labeled ‘Sealant’. Coat the third tile with oxalic acid, and place it in a beaker labeled ‘Calcium oxalate’.
4. The next day, measure 1200ml of vinegar with the measuring cylinder and pour 400ml into each of the 3 beakers. The vinegar solution will be used to simulate acid rain.
5. At the end of the day, remove and wipe the tiles down lightly, and record their weights in the table, under ‘Day 1’.
6. Repeat step 5 every day for the next 4 days, recording the new weights of the tiles in the table.
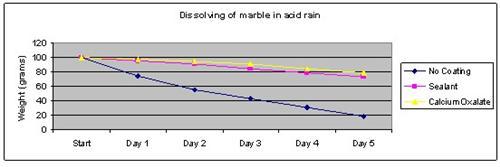
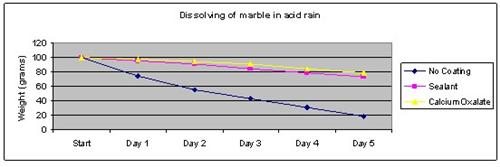
Results
The marble tile coated with calcium oxalate dissolved the least in the vinegar solution.
| Coating | Dissolving rate of marble tile in acidic solution (in grams) | |||||
| Start | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| No coating | 100 | 75 | 56 | 43 | 31 | 19 |
| Sealant | 100 | 96 | 91 | 85 | 79 | 73 |
| Calcium Oxalate | 100 | 98 | 95 | 91 | 85 | 79 |
The above results were then plotted onto a graph.
Conclusion
The hypothesis that the marble surface coated with calcium oxalate will show the least amount of decay has been proven to be true.
Acid rain is causing serious damage to buildings, monuments and sculptures that have a lot of historical value. Although there are commercial sealants and methods available that can protect marble surfaces, they are not suitable for use on the surfaces of fine work such as sculptures. The strong resistance to acid rain afforded by applying oxalic acid on statues is currently being studied in Italy.
Also consider
Will the results differ if the science fair project was repeated using different methods of applying the coating of oxalic acid (e.g. spraying, dipping and polishing)?
The science fair project may also be repeated by using limestone instead of marble.
References
How to affect acid rain in buildings? - http://worldacidrainsecrets.blogspot.com/2010/01/how-to-affect-acid-rain-in-buildings.html
How dies acid precipitation affect marble and limestone buildings? - http://pubs.usgs.gov/gip/acidrain/5.html
Calcium oxalate – a surface treatment for limestone - http://www.ucl.ac.uk/archaeology/conservation/jcms/issue4/cezar.html

