
Electrolysis: A Science Project
Hard
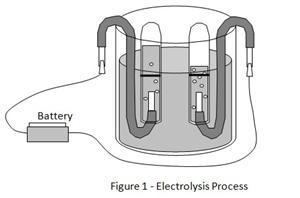
Have you ever wondered how electricity can be used to create gas? In this project, you'll explore the process of electrolysis and discover how to use electricity to create hydrogen gas.
Hypothesis
The hypothesis is that a higher DC voltage applied to the electrolysis process will increase the rate at which hydrogen gas is produced.
Method & Materials
You will use a beaker, tap water, salt, batteries, test tubes, and other materials to conduct the experiment.
You will need a beaker, tap water, salt, batteries, test tubes, and other materials.
Results
The results of this experiment show that increasing the DC voltage will result in a faster release of gasses from the chemical reaction in the electrolyte. However, increasing the amount of salt in the electrolyte does not change the rate of gas released from the electrolysis process.
Why do this project?
This science project is interesting because it explores the process of electrolysis and how electricity can be used to create gas.
Also Consider
Consider experimenting with different types of electrolytes such as vinegar or sulfuric acid. Also, examine the effects of raising or lowering the temperature of the solution during the process of electrolysis.
Full project details
You can find additional information and details for this science fair project here. Have fun exploring!Related videos
Hey there! Here are some awesome videos about this science project that we think you'll really like. They're not only super fun, but they'll also help you learn more about the science behind the project. So sit back, relax, and get ready to have some fun!!
Share this Science Project:
Related Science Fair Project Ideas
Have you ever wondered what happens when you add salt or sugar to water? Find out in this science project!
Hard
Learn how to extract metals from ore using displacement reactions! See which metals are more reactive and which are less reactive.
Hard
Test which metal is the most resistant to corrosion by immersing them in different solutions!
Hard
Share this Science Project:
