| Complexity level: | 7 |
| Project cost ($): | 10 |
| Time required: | 1 hour to prepare, 2 hours for the science project experiment |
| Material availability: | May be purchased at a chemist |
| Safety concerns: | Basic safety requirements |
Hypothesis
The Alka-Seltzer antacid tablet will dissolve most quickly in a hydrochloric acid solution and at the highest temperature.
Overview
Dissolving Alka-Seltzer
Alka-Seltzer is an antacid medication used for relief of heartburn and neutralizing stomach acid. The pH level in our stomach is normally between 2 to 3. The food that we eat will enter the stomach where acid is then secreted to help in its digestion. When too much food is eaten, even more acid is produced. At times, the pH level falls below 2. This is the cause of the heartburn.
Alka-Seltzer tablets consist of aspirin, sodium bicarbonate and citric acid. As an antacid the tablet will provide quick pain relief and help to raise the pH level in the stomach to between 3 or 4.
Alka-Seltzer medication is taken by dissolving the tablet in water and drinking it. Once the tablet is placed in the water, the citric acid will dissolve in water, making it acidic. The acidic solution will react with the sodium bicarbonate and release carbon dioxide. The releasing of carbon dioxide gas will create fizzy bubbles in the drink. The mixture is then drunk to reduce stomach discomfort and to neutralize stomach acids.
Scientific Terms
Materials
The materials required for this science fair project:
- 8 Alka-Seltzer tablets
- 8 beakers
- 1 large beaker
- 1 bottle of ice water
- 1 pack of ice cubes
- 800ml of Hydrochloric acid at pH 2
- 1 hot plate
- 1 thermometer
- pH paper
- 1 measuring cylinder
- 1 stopwatch
Procedure
1. For this science project, the independent variable is whether water or hydrochloric acid is used as the solution, and the temperature of the solution. The dependent variable is the time taken by the Alka-Seltzer tablet to fully dissolve in the solution. This is determined using a stopwatch. The constants (control variables) are the amount of water used, the size of the tablet and the air pressure in the room.
2. The first beaker is filled with 200ml of ice water. Water and ice cubes are added to bring the temperature to 15°C, and the volume of the water is adjusted back to 200ml. 1 Alka-Seltzer tablet is placed in the water and the time taken for the tablet to fully dissolve is checked using the stopwatch and recorded in the table given below.
3. Using another 3 beakers filled with 200ml water and the hot plate, the temperature of the water is brought to 25°C, 35°C and 45°C. One Alka-Seltzer tablet is placed in each beaker and the time taken for the tablet to dissolve is measured and recorded in the table below.
4. The remaining 4 beakers are filled with hydrochloric acid. The temperature of the first beaker is brought to 15°C as explained in procedure 2 and the time for the Alka-Seltzer tablet to dissolve is measured and recorded in the table below.
5. The temperature of the remaining 3 beakers filled with hydrochloric acid are brought to 25°C, 35°C and 45°C using the hot plate and the time required for the Alka-Seltzer tablet to dissolve is measured and recorded in the table below
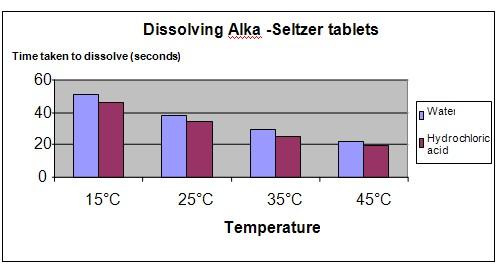
Results
The results show that as the temperature of the solution increases, the Alka-Seltzer tablet dissolves more quickly. The tablet also dissolves more quickly in acidic solutions, compared to water.
|
Substance |
Time for Alka-Seltzer tablet to dissolve (seconds) |
|||
|
15°C |
25°C |
35°C |
45°C |
|
|
Water |
51 |
38 |
29 |
22 |
|
Hydrochloric acid |
46 |
34 |
25 |
19 |
The chart below represents our experiment results.
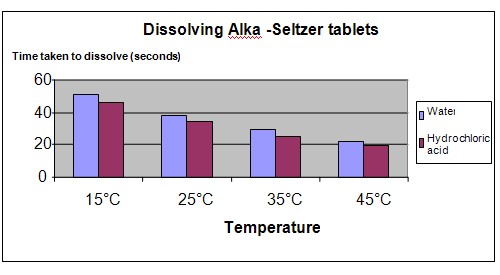
Conclusion
The hypothesis that Alka-Seltzer antacid tablets will dissolve most quickly in hydrochloric acid solution and at the highest temperature, is proven to be true.
Antacids are used for quick relief from heartburn and stomach discomfort especially after a heavy meal. In order to see quick results, the tablets are best taken with warm water so that they dissolve most quickly. Most mediations are in fact taken best with warm water rather than cold water.
Also consider
Try to repeat the science fair project by using other tablets like vitamin C.
The science fair project can also be repeated using an acidic solution to observe how long it takes the acidity to be neutralized by the antacid tablet.
References
Alka-Seltzer - http://en.wikipedia.org/wiki/Alka-Seltzer
Why does Alka-Seltzer fizz? - http://science.howstuffworks.com/question116.htm

