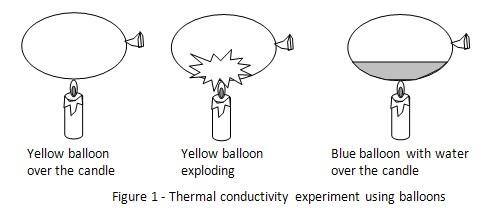
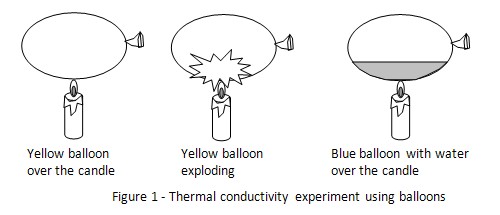
Hypothesis
A balloon filled with water will be able to withstand fire.
Overview
Thermal conduction
Thermal conduction is the ability of a medium to allow heat to be conducted through it. The heat will always travels from a hotter spot to a cooler spot. For example, if one end of a metal rod is heated up, the heat will gradually travel to the other end until the entire rod becomes hot. Thermal conduction exists in solids, liquids and gasses.
The conduction of heat through a medium happens because the particles in the medium vibrate or move faster when heat is applied to it. This vibration or movement of the particles will start at the point where the heat originates. As the particleenergy and vibration increase, they will collide with the neighboring particles and transfer thermal energy to them. When this happens the temperature of the original particle will drop and the temperature of the neighboring particle will increase. However the original particle will replenish the energy again from the heat source. This process will continue until the entire medium reaches thermal equilibrium where the temperature is the same everywhere in the medium.
Thermal conduction can also take place between different mediums that are at different temperatures. An example is boiling water in a metal pot. The stove is the source of the heat. The fire from the stove will heat up the metal pot. As the temperature of the pot increases and its particles start to vibrate more quickly, the heat is transferred to the water in the pot and the water molecules increase their movement and collisions with other water molecules. This will go on until the water in the pot begins to boil.
Scientific Terms
Thermal conduction, solid, liquid, gas, thermal equilibrium
Conclusion
The hypothesis that a balloon filled with water will be able to withstand fire is proven to be true.
Most of the balloons are made from rubber which has a thermal conductivity of 0.16 W•K-1•m-1. The yellow colored balloon was filled with only air which has a thermal conductivity of 0.025W•K-1•m-1. Therefore the balloon surface over the candle was not able to transfer much heat to the air inside the balloon. The rubber surface became hot, melted and the balloon exploded.
The blue colored balloon had water and air inside it. Water has a thermal conductivity of 0.6 W•K-1•m-1 .The water was able to absorb the heat on the balloon surface when it was held over the candle. The temperature of the balloon surface was kept low and it did not explode.
Also consider
Try a different experiment by placing the inflated balloon in hot water and cold water to observe the changes.
References
Thermal conductivity - http://en.wikipedia.org/wiki/Thermal_conductivity
What is thermal conduction? -http://www.wisegeek.com/what-is-thermal-conduction.htm
Related videos
These videos explain the science behind this project and demonstrate key concepts used in the experiment.