| Complexity level: | 6 |
| Project cost ($): | 20 |
| Time required: | 1 hour to prepare, 1 day for the science project experiment |
| Material availability: | Easily found |
| Safety concerns: | Always handle boiling water with extreme care to avoid getting burned. Adult assistance is required. |
Hypothesis
Water will come to boil more quickly at lower temperatures at higher altitudes.
Overview
Boiling Point
A liquid will start to boil when the temperature of the liquid reaches a point where the pressure of the vapor from the liquid equals the pressure of the surrounding air. A liquid placed in vacuum will boil at lower temperatures than in normal conditions. Different liquids will have different boiling temperatures.
When water is boiled at higher altitudes above sea level, it will boil at lower temperatures. For example, water will boil at only 69°C on top of Mount Everest compared to 100°C at sea level. This is because, at higher altitudes, the air pressure is lower and therefore evaporation happens at lower temperatures.
Adding salt, sugar or other substances to water will usually increase its boiling temperature. This will be useful, for example, when boiling you need to boil water at higher altitudes and would like to attain a higher temperature to ensure that microorganisms in the water are killed.
Scientific Terms
Materials
The materials required for this science fair project:
- 1 high rise building at least 50 storeys high
- 1 hot plate
- 1 stove
- 8 beakers
- 1800ml water
- 1 air-conditioned room
- 1 thermometer
Procedure
1. For this science fair project, the independent variables are the conditions for boiling the water - different amounts of water, diffenent types of heating devices, different room temperatures and different floor level of a building. The dependent variable is the boiling temperature of water. This is determined by measuring the temperature using the thermometer. The constants (control variables) are the beaker used to contain the water, the impurities in the water and the heating capacity of the stove and hot plate.
2. The science project experiment is done in 4 stages to compare the boiling point of water :
a. using different amounts of water,
b. using different types of heating devices,
c. using different room temperatures,
d. test at different floors of a high rise building to ensure that the air pressure is different.
3. For the 1st experiment, 2 beakers are used. The amount of water poured into each beaker is 200ml and 400ml. The water is boiled on a hot plate and the boiling temperature is recorded . The measurements are recorded in the table below.
4. For the 2nd experiment, 2 beakers are each filled with 200ml water. One of the beakers is heated up on the hot plate and the other beaker is heated on the stove. The boiling temperatures are measured and recorded in the table below.
5. For the 3rd experiment, another 2 beakers are filled with 200ml water each. The room temperature is brought to 18°C and the water is boiled on the hot plate and the temperature is recorded. The room temperature is then adjusted to 28°C and the water in the 2nd beaker is brought to boil on the hot plate and the temperatures are recorded in the table below.
6. The 4th experiment is carried out in a high rise building. 200ml water is filled into the 1st beaker and brought to a boil using the hot plate, on the ground floor. The 2nd beaker is filled with 200ml water and taken to the 50th floor of the building and brought to boil using the hot plate. Both temperature measurements are recorded in the table below.
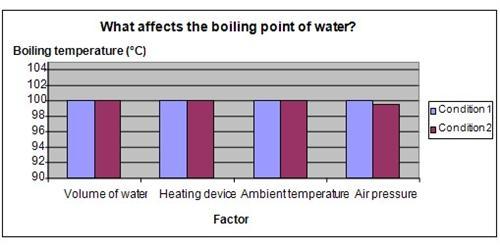
Results
It is observed that the water boiled at 0.5 ° C lower on the 50th floor of the building. For all the other conditions, the boiling point of water was the same.
|
Condition |
Description |
Water boiling temperature |
|
|
Volume of water |
1 |
200ml water |
100° C |
|
2 |
400ml water |
100° C |
|
|
Heating device |
1 |
Hot plate |
100° C |
|
2 |
Stove |
100° C |
|
|
Ambient temperature |
1 |
Room 18 ° C |
100° C |
|
2 |
Room 28 ° C |
100° C |
|
|
Air pressure |
1 |
Ground floor |
100° C |
|
2 |
50th floor |
99.5° C |
The chart below represents the results of our science experiment.
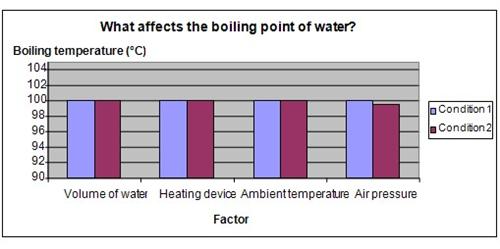
Conclusion
The hypothesis that water will come to boil more quickly at lower temperatures at higher altitudes, has been proven to be correct.
Also consider
What would happen if the science fair project were to be repeated on a rainy day instead of a hot sunny day?
The science project can also be modified to observe the effects of different concentrations of salt on the boiling temperature of water.
References
Boiling point - http://en.wikipedia.org/wiki/Boiling_point
How to boil water? - http://whatscookingamerica.net/boilpoint.htm

