| Complexity level: | 2 |
| Project cost ($): | 5 |
| Time required: | 1 hour to prepare, 4 hours for experiment |
| Material availability: | Easily found |
| Safety concerns: | None |
Hypothesis
Evaporation of water will happen faster given a higher surface area, higher temperature and lower air pressure environment.
Overview
Evaporation
Evaporation is the process by which liquid spontaneously turns into gas. In the case of water, it turns into water vapor.
Water molecules need energy to evaporate and become water vapor. The molecules inside the water have kinetic energy, which are in a continuous state of motion, shifting and colliding with each other. There will be a transfer of energy between the molecules colliding amongst themselves. At times, energy transferred to a water molecule located near the surface is sufficient for the molecule to escape and become water vapor.
When the surface area of the water is large, there will be a larger number of water molecules located near the surface area of the water and hence, water molecules will turn into water vapor at a faster rate.
Warmer temperatures cause the water molecules to have a higher amount of kinetic energy and will therefore produce a higher frequency and magnitude of collisions between water molecules. This leads to a faster rate of evaporation. Boiling water will evaporate at a faster rate because of the same reason.
At a lower atmospheric pressure, less energy is needed by the water molecules to escape into the atmosphere. Therefore more molecules at the water surface will be able to reach the required energy level and evaporation will occur at a faster rate.
Scientific Terms
Materials
The materials required for this experiment:
- 5 plates that can hold 100ml water
- 1 glass that can hold 100ml water (the area of the mouth of the glass must be at least 3 times smaller than the plate)
- 600 ml water
- 1 fan
Procedure
- For this experiment, the independent variables are the water- surface area, temperature and air pressure. The dependent variable is the rate of water evaporation. The constants (control variables) are the amount of water used in each experiment.
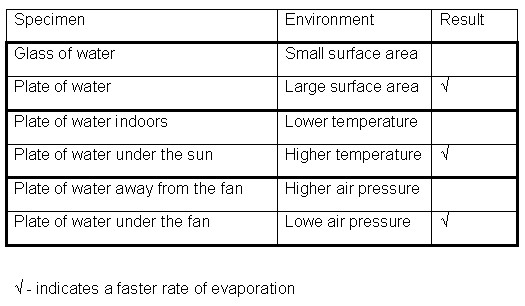
- The glass and one of the plates are filled with 100ml of water each. The water in the plate will have a larger surface area than the water in the glass. The rate of evaporation is observed every 10 minutes. The results are recorded in the table below.
- Two of the plates are filled with 100ml of water each. One of the plates is placed indoors where the temperature is cooler and the other plate is placed directly under the sun where the temperature is higher. The rate evaporation of the water is observed every 10 minutes. The results are recorded in the table below.
- The last two plates are filled with 100ml of water each. One of the plates is placed directly in the pat of the wind from the fan and the other plate is placed away from the fan. The wind from the fan will reduce the air pressure around the first plate. The rate of evaporation of the water is observed every 10 minutes. The results are recorded in the table below.

Results
The results show that the plate with the larger space area, the plate placed under the sun and the plate placed under the fan had the water evaporating faster.
Conclusion
The hypothesis that the rate of evaporation of water will be faster with a larger surface area, higher temperature or lower air pressure environment, is proven to be true.
Evaporation is part of the water cycle. Water from the oceans evaporates and forms clouds in the sky. The wind blows these clouds towards the land where the water precipitates and falls as rain on to dry land. This water is collected in rivers and lakes as fresh water that we will eventually be used as drinking water. River waters flow back into the ocean and the process repeats itself.
Also consider
The experiment can also be repeated using oil, alcohol and other liquids to compare the rate of evaporation.
References
- Evaporation - http://en.wikipedia.org/wiki/Evaporation
- Evaporation - http://www.browseinfo.net/evaporation.html

