| Complexity level: | 6 |
| Project cost ($): | 40 |
| Time required: | 2 hour to prepare, 1 day for observation |
| Material availability: | Easily found at a hobby store. Access to a laboratory is required |
| Safety concerns: |
Hypothesis
Batteries will supply power for a shorter length of time when temperatures fall.
Overview
Batteries
Batteries operate by storing energy in the form of chemicals and converting them into electricity when the terminals are connected to a device. Batteries are available in many different voltages and sizes. They are also available as disposable batteries or rechargeable batteries.
Batteries are constructed with a positive electrode called a cathode and a negative electrode called an anode. These two electrodes are not directly connected to each other but placed in a medium called an electrolyte. When the terminals of the electrodes are connected to a load or device, a chemical reaction will take place in the medium whereby the anode will "supply" electrons to the cathode. These electrons travel through the electrolyte.
The performance and life of the battery is affected by temperature and humidity. Batteries produce electricity through chemical processes. When temperatures drop, the chemical reaction in the electrolyte will occur more slowly resulting in smaller currents produced. However, the battery will be able to last longer. In extremely cold conditions, the electrolyte might freeze over. Increasing the temperature will result in faster chemical reactions, but the battery will also run out of power faster.
Scientific Terms
Materials
The materials required for this science fair project:
- 3 Energizer AA size batteries
- 3 Duracell AA size batteries
- 3 Eveready AA size batteries
- 6 plastic bags
- 6 rubber bands
- Battery holder
- 2 jumper wires with crocodile clips
- 1 AA battery- operated table fan
- 2 beakers
- 1 kg of dry ice
- 1 kg of ice cubes
- Safety goggles
- 1 pair of thick waterproof gloves to be used to handle the dry ice
- A clock to time the duration of the fan’s operation
MEL Physics — monthly physics experiment kits delivered to your door. (Affiliate link)
See what’s includedProcedure
1. For this science fair project, the independent variables are the type of the battery and the temperature conditions. The dependent variable is the time taken for the battery to run out of power while turning the fan motor. This is determined by keeping time with the clock. The constants (control variables) are the power of the fan motor, the size of the batteries and room humidity.
2. The performance of the batteries will be tested at the following temperatures: -78°C using dry ice, 0°C using ice cubes and 24°C at room temperature.
3. For the 1st test, an Energizer battery is placed in the battery holder, and 2 jumper wires with crocodile clips are connected to the holder. The battery and the holder are placed in a plastic bag with the other end of the jumper wires sticking out of the bag. The plastic bag is tied with the rubber band.
4. The plastic bag with the battery is placed in a beaker. Wear the gloves and transfer the dry ice to the beaker. Remember not to let the dry ice come into contact with your bare skin. Wear safety goggles at all times. Allow about 15 minutes for the temperature of the battery to stabilize in the beaker. The fan is then connected to the 2 wires sticking out of the plastic bag. Once the fan turns, the clock is started. With the help of an assistant, the time taken for the battery to drain and the fan to stop is recorded in the table given below.
5. Procedures 3 and 4 are repeated using the Duracell and Eveready batteries.
6. Procedures 3, 4 and 5 are repeated by using ice cubes instead of dry ice.
7. Procedures 3, 4 and 5 are repeated at room temperature without any cooling needed.
8. All the measurements recorded in the table given below.
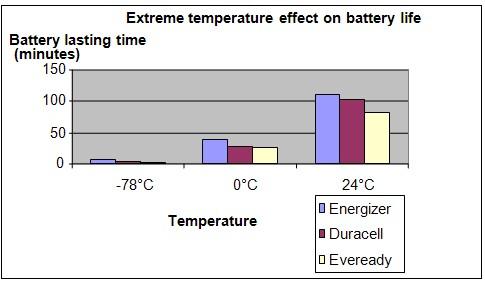
Results
It is observed that the maximum operation time of the batteries become shorter as the temperature falls. At a temperature of -78°C, the batteries only lasted a few minutes.
| Battery | Time taken for the fan to stop (minutes) | ||
| -78°C | 0°C | 24°C | |
| Energizer | 6.7 | 38.5 | 112.3 |
| Duracell | 4.8 | 28.3 | 103.7 |
| Eveready | 2.9 | 24.9 | 81.2 |
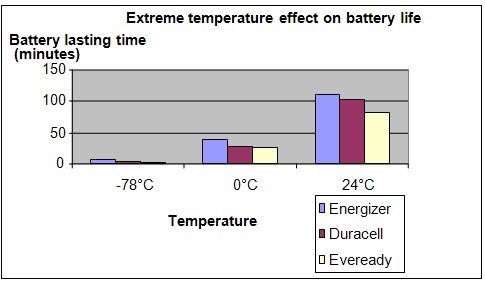
Conclusion
The hypothesis that a battery will supply power for a shorter length of time when its temperature falls, is proven to be true.
Batteries are used to operate small portable electrical devices like watches, calculators, torch lights, cell phones and many more. They are also very useful for use in remote areas without wired electricity or when we experience power disruptions at home. Understanding how batteries perform under different temperature conditions helps us properly plan for our power requirements.
Also consider
What would happen if this science fair project were to be repeated at higher temperatures of 40°C, 50°C, 60°C, instead
The experiment can also be repeated using rechargeable batteries, such as Nickel Metah Hydride batteries commonly used in mobile phones and cameras.
References
Battery Electricity - http://en.wikipedia.org/wiki/Battery_ (electricity)
Temperature affects batteries - http://www.batteryeducation.com/2006/08/temperature_aff.html

