Hypothesis
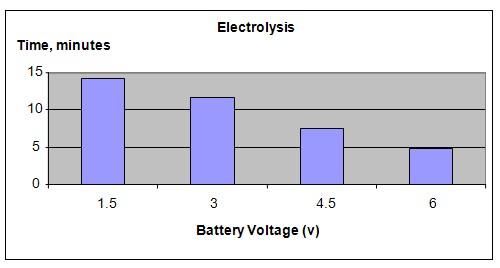
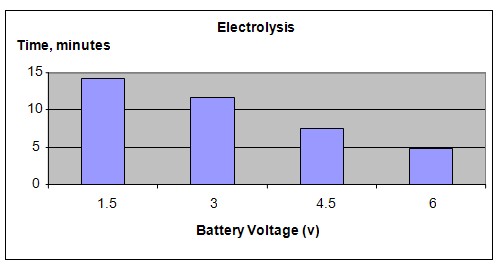
Increasing the DC voltage applied to an electrolyte solution will increase the rate of release of hydrogen gas.
Overview
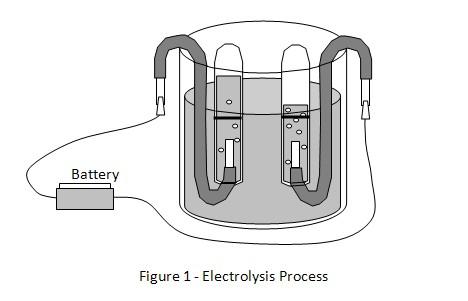
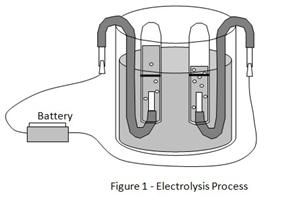
Electrolysis process
Electrolysis refers to a chemical process that occurs in an electrolyte solution when an electric current is passed through the solution. An electrolyte solution is made up of negatively charged ions - cations - and positively charged ions - anions. Cations and anions move freely in an electrolyte solution.
The electrolysis process takes place when the positive and negative terminals of a battery are connected to conductors called electrodes and immersed in the electrolyte solution. The electrode connected to the negative terminal is called the cathode and the electrode connected to the positive terminal is called an anode. When the cathode and anode are connected to the battery and immersed in the electrolyte, the negative ions (anions) will be attracted to and move towards the anode (positive electrode). The positive ions (cations) will be attracted to and move towards the cathode (negative electrode).
When the ions reach the surface of the electrodes the following reactions may occur.
- Deposits may start to form on the surface of the electrode.
- Gasses may be released from the electrodes.
- The product of the electrolysis process may be dissolved in the electrolyte solution.
Scientific Terms
Electrolysis, electrolyte, ions, cations, anions, electrodes, anode, cathode
Conclusion
Our hypothesis has been proven to be true. Increasing the DC voltage applied to the electrolyte solution will speed up the release of hydrogen gas.
Electrolysis has many uses. It is used in industrial processes like electroplating as well as in certain measuring devices like the pH meter. Astronauts use electrolysis to produce oxygen in space.
Also consider
This science fair project can be repeated by varying the amount of salt in the water.
This science project may be modified by using different types of electrolytes like vinegar or sulfuric acid.
References
Electrolysis - http://en.wikipedia.org/wiki/Electrolysis
Electrolysis - http://www.tutorvista.com/content/chemistry/chemistry-iii/redox-reactions/electrolysis.php
Related videos
Hey there! Here are some awesome videos about this science project that we think you'll really like. They're not only super fun, but they'll also help you learn more about the science behind the project. So sit back, relax, and get ready to have some fun!!