| Complexity level: | 6 |
| Project cost ($): | 20 |
| Time required: | 2 hour to prepare, 3 days for the science project experiment |
| Material availability: | Easily found at a hardware or department store |
| Safety concerns: | None |
Hypothesis
Batteries will last longer at around room temperature (about 26°C). We hypothesise that manufacturers would design their batteries to perform optimally at room temperature, since most batteries would be used by consumers, in environments at room temperature.
Overview
Batteries
Batteries are devices that convert stored chemical energy in the battery into electrical energy when its terminals are connected. Batteries consist of rechargeable and disposable types. They can be found in various sizes and voltages.
Batteries consist of 2 electrodes (an anode which is negative and a cathode which is positive) and the electrolyte. The two electrodes do not have a physical connection but are electrically connected through the electrolyte. When the battery terminals are connected, a chemical process occurs in the electrolyte where electrons will be received from the anode and supplied to the cathode.
The performance of a battery depends on humidity and temperature. This is because the battery produces electricity through a chemical process. As the temperature becomes lower, the chemical reaction slows down and a smaller current is produced but the battery is able to last longer. As the temperature increases, the chemical reaction becomes faster and more current is produced but the life of the battery shortens.
Scientific Terms
Materials
The materials required for this science fair project:
- An air-conditioned room
- 3 Energizer AA size batteries
- 3 Duracell AA size batteries
- 3 Eveready AA size batteries
- 3 AA battery-operated table fans – ensure that they are of the same make and model
- A clock or stopwatch
- An assistant
Procedure
1. For this experiment, the independent variable is the type of battery and the room temperature. The dependent variable is the time taken for the battery to run out of power. This is determined by checking the time with the clock. The constants (control variables) are the power of the fan motor, the size of the batteries and the humidity in the room.
2. The performance of the batteries will be tested at room temperatures of 18°C, 22°C and 26°C.
3. On the 1st day of testing the room temperature is brought down to 18°C by adjusting the air conditioner. Once the temperature has stabilized, the 3 fans are marked with the battery names, i.e. Energizer, Duracell and Eveready.
4. The 3 fans are powered on and the clock is started. With the help of the assistant, the time taken for the battery power to run low till each of the fans stops, is recorded in the table given below.
5. On the 2nd day, the experiment is repeated by setting the room temperature at 22°C. Once the room temperature had stabilized, the procedure 4 is repeated and the time taken by the fans to stop is recorded in the table below.
6. Finally on the 3rd day of experiment the room temperature is adjusted to 26°C and procedure 4 is repeated again.
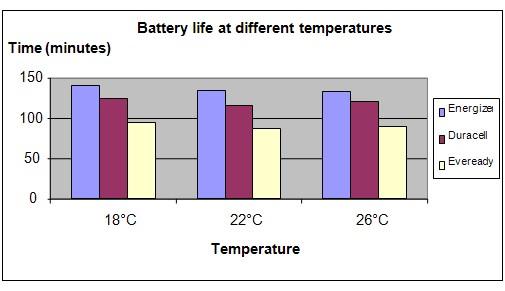
Results
It is observed that there is not much difference in the durability of the battery as the temperature changes. However it was found that the Energizer battery lasted the longest and the Eveready battery was the fastest to go flat.
|
Battery |
Time taken for the fan to stop (in minutes) |
||
|
18°C |
22°C |
26°C |
|
|
Energizer |
142 |
134 |
132 |
|
Duracell |
124 |
116 |
120 |
|
Eveready |
95 |
88 |
80 |
The chart below represents the results of our experiment.
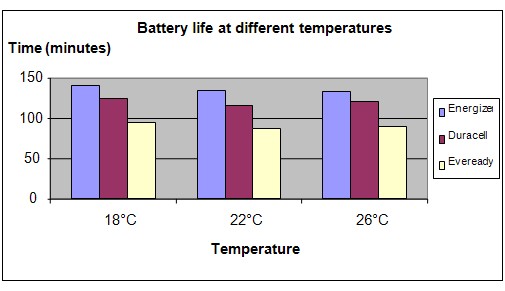
Conclusion
The hypothesis that batteries will last the longest at around room temperature (about 26°C) is not proven to be true. We hypothesized that the performance of batteries will be affected by changing temperatures but in this experiment the differences in the temperature was perhaps too small to observe the changes.
Batteries are used to operate small portable devices like torch lights, watches and cameras. They help to operate small devices in places where electricity supply is not available.. However, the disposal of the batteries is a problem because they contain chemicals that are not environmentally-friendly.
Also consider
What would happen if the experiment were to be repeated at a larger range of different temperatures from 0°C to 80°C?
The experiment can also be repeated by using rechargeable batteries.
References
Source for Overview:
Battery Electricity - http://en.wikipedia.org/wiki/Battery_ (electricity)
Temperature affects batteries - http://www.batteryeducation.com/2006/08/temperature_aff.html

