| Complexity level: | 9 |
| Project cost ($): | 80 |
| Time required: | 1 hour to prepare, 1 hour for the science project experiment |
| Material availability: | May be found at a hardware store, and a chemist |
| Safety concerns: | Avoid long, unprotected exposure to the sun. Handle glass extremely carefully. Adult assistance required. |
Hypothesis
Higher concentrations of Octyl salicylate in soap mixtures are able to block a greater amount of UV radiation.
Overview
Sunscreen
Sunscreen lotions or soaps are used to block, absorb or reflect ultraviolet radiation from the sun. They are applied to our skin for protection from the sun’s ultraviolet rays and help in the prevention of sunburn.
The effectiveness of a sunscreen is measured by their Sun Protection Factor (SPF) rating. A higher SPF rating generally means greater protection from the sun’s UV-B radiation (the ultraviolet ray that causes skin sunburn). The amount of protection from UV-B radiation will depend on our skin type, the amount of sunscreen applied, the amount of sweat or type of activity involved and the amount of sunscreen absorbed by the skin.
Sunscreens are made using substances that filter ultraviolet rays. These materials are organic compounds, organic particulates or inorganic particulates. Octyl salicylate is an organic compound that is used to absorb UV-B radiation from the sun. It is a colorless and oily liquid.
UV index
The simplest way of measuring ultraviolet radiation is the UV index. Each unit on the UV index represents about 25mW per square meter of UV radiation. The UV index range is described as follows:
Less than 3: Moderate levels
Between 3 and 6: High levels - enough to cause sunburn even at temperatures below 27 degrees Celsius
Between 7 and 9: Very high levels - enough to cause sunburn on cloudy days
More than 9: Extreme levels - that can cause sunburn to an unprotected skin within 12 minutes.
Scientific Terms
Materials
The materials required for this science fair project:
- 5 glass sheets measuring 500mm x 500mm
- 1 UV meter to measure UV index
- 1 wooden box with open top (approximately 500mm length x 500mm width)
- 500 ml alcohol
- 250 gram of glycerin
- 10 gram of Octyl salicylate
- 5 beakers
- 1 measuring cylinder
- 5 sponges
- 1 spatula
- 1 digital weighing scale
Procedure
1. For this science fair project, the independent variable is the concentration of Octyl salicylate in the soap solution. The dependent variable is the level of the UV index measured. This is determined with the UV meter. The constants (control variables) are the time the readings are taken, the intensity of sunlight and other weather conditions on the day of testing and the location where the testing is done.
2. The 5 sheets of glass and 5 beakers are labeled as 0%, 2%, 4%, 6% and 8%. Using the measuring cylinder, 100ml of alcohol is poured into each beaker. 50 mg of glycerin in also added to each beaker.
3. In the beaker labeled as 0%, no Octyl salicylate is added. In the beaker labeled 2% - 1 gram Octyl salicylate, 4% - 2 grams Octyl salicylate, 6% - 3 grams Octyl salicylate and 8% - 4 grams Octyl salicylate is added. The solution is mixed using the spatula.
4. Using separate sponges, the soap solution in the beakers is spread evenly over the glass surface according to the labels, and allowed to dry.
5. The wooden box is placed in a location where it receives direct sunlight without any obstruction or shadows. The UV meter is placed inside the box and the UV index reading is taken. The reading is recorded in the table given below.
6. The glass sheet with the 0% marking is placed over the box with the UV meter still inside the box. The new UV index is observed and recorded in the table given below.
7. Procedures 5 and 6 are repeated using the glass sheets with 2%, 4%, 6% and 8% markings. All of the UV index readings before and after placing the glass over the box are observed and recorded in the table given below.
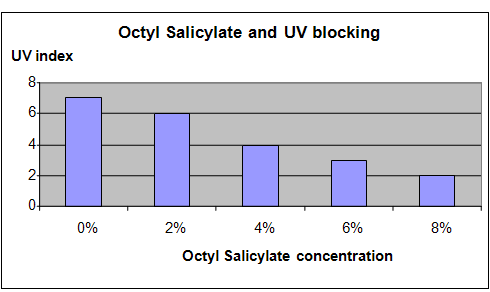
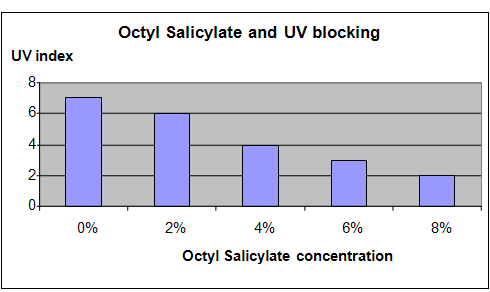
Results
It was observed that increasing the concentration of the Octyl Salicylate in the soap used to coat the glass surface resulted in increasingly lower UV index readings. This means that increased concentrations of Octyl Salicylate resulted in more effective blockage of UV radiation.
|
Condition |
Octyl Salicylate concentration and UV index readings |
||||
|
0% |
2% |
4% |
6% |
8% |
|
|
Without glass |
7 |
7 |
7 |
7 |
7 |
|
With glass |
7 |
6 |
4 |
3 |
2 |
The chart below represents the results of our science project experiment.
Conclusion
The hypothesis that higher concentrations of Octyl salicylate in a soap mixture would result in greater blockage of UV radiation, has been proven to be true.
The use of sunscreens will block the UV-B and prevents sunburn but it may not necessarily be able to block the UV-A radiation. People who use sunscreen regularly risk overexposure to UV-A and increase their chance of getting a kind of skin cancer called melanoma. Moreover, using the sunscreen too often can also result in vitamin D deficiency.
Also consider
Would the results of our science fair project differ if it were to be repeated using different types of sunscreen chemicals like oxybenzone or zinc dioxide?
The science project experiment can be also repeated using a UV lamp and performing the experiment indoors, for a more regulated amount of UV radiation.
References
Sunscreen - http://en.wikipedia.org/wiki/Sunscreen
Octyl salicylate - http://en.wikipedia.org/wiki/Octyl_salicylate
Why measure UV? - http://www.solatell.com/Why%20measure%20UV.htm
How to measure the UV index using the UV meter? - http://www.bom.gov.au/info/weatherkit/section2/pdf/uv.pdf

