| Complexity level: | 7 |
| Project cost ($): | 30 |
| Time required: | 1 hour for preparation, 1 hour for observation |
| Material availability: | Easily found. Iodine solution may be obtained from a chemist |
| Safety concerns: |
Hypothesis
Peanut oil will have the highest proportion of saturated fat, and when the iodine test is performed, will require the longest time to lose its purple color.
Overview
Cholesterol and saturated fat
The two types of cholesterol in our body are known as Low Density Lipoproteins (LDP) and High Density Lipoproteins (HDP). The unhealthy cholesterol, LDP, increases the risk of heart disease while the healthy cholesterol, HDP, lowers that risk. As the consumption of saturated fats is likely to increase the levels of LDP in our bodies, using cooking oil with a lower level of saturated fat can provide for better health.
Saturated fat is normally found in foods such as butter, fatty meat, cheese, cream, chocolate and pie. To lower the intake of saturated fat and reduce one’s cholesterol levels, it is better to consume lean meat and low-fat dairy products instead of their higher-fat alternatives. Healthier substitutes for saturated fats are polyunsaturated or monounsaturated oils, which can be found in olive oil, fish oil and cornflower oil. In particular, eating fish for their Omega-3 polyunsaturated fat content can protect the blood arteries by preventing blood clots.
Besides consuming less saturated fat, regular exercise can also help to reduce the level of cholesterol in our blood.
Scientific Terms
Materials
The materials required for the science fair project:
- 1 bottle of peanut oil
- 1 bottle of canola oil
- 1 bottle of corn oil
- 1 bottle of soya bean oil
- 1 bottle of olive oil
- 5 test tubes
- 1 test tube stand
- 1 eyedropper
- 1 bottle of iodine
- 1 stopwatch
- 1 measuring cylinder
MEL Chemistry — monthly chemistry experiment kits delivered to your door. (Affiliate link)
See what’s includedProcedure
1. For this science fair project, the independent variable is the type of oil used for testing - peanut oil, canola oil, corn oil, soya bean oil and olive oil. The dependent variable is the time taken for the iodine color to clear when the iodine test is performed, and will be measured with a stopwatch. The constants (control variables) are the amount of oil used for testing, the number of drops of iodine used and the temperature of the environment, which remained at room temperature.
2. Clean the 5 test tubes were and arrange them in the test tube rack. Label them according to the oils used for testing - peanut oil, canola oil, corn oil, soya bean oil and olive oil.
3. Measure 20ml of each type of oil with the measuring cylinder, and pour into the respective test tubes.
4. Drip 3 drops of iodine into the first test tube. Start the stopwatch immediately and record the time taken for the purple color of the iodine to disappear, as shown below.
5. Repeat Step 4 on the 4 types of oil, and record the results in a table.
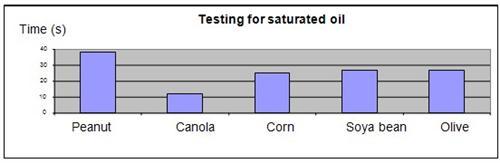
Results
It was observed that the iodine and peanut oil mixture took the longest to revert to the original color, while the canola oil took the shortest amount of time.
| Oil type | Cooking oil type, content and time for iodine color to clear (in seconds) | ||||
| Peanut | Canola | Corn | Soya bean | Olive | |
| Saturated oil % | 18% | 7% | 13% | 15% | 14% |
| Time for iodine to clear | 38 | 12 | 25 | 27 | 27 |
The results were plotted onto a graph, as shown below.
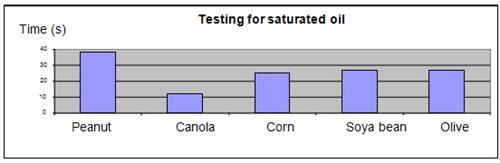
Conclusion
The hypothesis that peanut oil will have the highest proportion of saturated fat, and will take the longest time to become revert to its original color, was proven to be true. Peanut oil has the highest saturated fat content and the lowest proportion of unsaturated fat among the 5 types of cooking oil tested. As iodine reacts with unsaturated oil to lose its color, a lower proportion of unsaturated oil content will require a longer reaction time for the iodine in the oil to lose its color.
The consumption of unsaturated fat instead of saturated fat is recommended to maintain a healthy heart, as well as to reduce the risk of stroke and cancer. Oils that are low in saturated fat and high in unsaturated fat should be used in cooking. Consuming more soluble fiber that is found in fruits and vegetables can help to reduce the cholesterol levels in the blood.
Also consider
What would happen if the science fair project was repeated using different types of oils like oil palm, coconut oil or sunflower oil?
The science fair project can be also repeated to measure the amount of iodine required for all the unsaturated fat in the oil to react.
References
Best cooking oil, healthy cooking oils and olive oil - http://www.fatfreekitchen.com/cholesterol/cookingoil.html
How to reduce your cholesterol level - http://www.ehow.com/how_2280843_reduce-cholestrol-level.html

