| Complexity level: | 9 |
| Project cost ($): | 80 |
| Time required: | 1 day for preparation, 2 days for experiment |
| Material availability: | You will need access to specific laboratory equipment |
| Safety concerns: |
Hypothesis
The soybean peroxidase can be purified by liquid chromatography
Overview
Soybean peroxidase
Soybean peroxidase is the latest addition to the arsenal of medical tools that are now being used in diagnosing AIDS. When geneticist Rick Vierling from Purdue University started to do research on how to add value to soybeans, he had not expected that his research would be able help doctors in China to diagnose AIDS in patients. New medical diagnostic kits that use soybean peroxidase-based compounds are slated to be released into the market.
Previously, horseradish peroxidase was used in medical kits to help with diagnosing bacterial, viral and parasitic diseases such as malaria and AIDS. Manufacturers of these medical kits are now interested in soybean peroxidase because of its abundance of supply as compared to horseradish. Moreover, horseradish peroxidase enzyme is not very stable at high temperatures.
Standard medical diagnostic kits are said to lose their effectiveness within 4 months if they are stored without any refrigeration. The new medical diagnostic kits based on soybean peroxidase enzymes are able to last for up to a year when stored without refrigeration. This makes them suitable for use in countries with warmer climates, such as Africa, South America, India and China.
Soybean peroxidase has high thermal stability and is able to withstand temperatures between 20 degrees C and 90 degrees C without becoming denatured. It is also stable between pH 2.0 and pH 8.0 and will produce peak catalytic activity when the pH is 2.4. These are 2 characteristics of soybean peroxidase that make it stand out compared to other enzymes, which will denature under such high temperatures.
Scientific Terms
Materials
The materials required for the science fair project:
- 1 bottle of ethanesulfonic acid
- 1 packet of sodium chloride
- 1 bottle of pyrogallol
- 1 bottle of hydrogen peroxide
- 1 bottle of potassium phosphate
- 1 bottle of soybean peroxidase
- 1 unit of the BioCad Perfusion Sprint Chromatography system
- 1 anion exchange polymer (10mm x 50mm)
- 1 YM30 Amicon ultrafiltration disc
- 1 bottle of distilled water
- 1 Virtis Vacfreeze refrigerated condenser module
Procedure
1. For this science fair project, the independent variable is the level of catalytic activity of the commercial and purified soybean peroxidase. The dependent variable is the catalytic activity of the soybean peroxidase. This is determined by the colorimetric measurement of oxidizing pyrogallol to purpurogallin. The constants (control variables) are the pH, amount of sodium chloride and temperature of the environment, which will remain at room temperature.
2. Obtain the commercial soybean peroxidase and purify it using the BioCad Perfusion Sprint Chromatography equipment and the anion exchange polymer (10mm x 50mm).
3. Perform the ultrafiltration to remove salt by using the YM30 Amicon ultra filtration disc. Wash the purified soybean peroxidase protein 6 times using the distilled water. Next, lyophilize the soybean peroxidase protein overnight using a Vitris Vacfreezer.
4. Test and compare the catalytic activity of the purified soybean peroxidase with the original commercial soybean peroxidase. Use a 0.01M potassium phosphate as a buffer at pH 6.0 together with 7.84M hydrogen peroxide at 20 deg C to oxidize 42.3 mM soybean peroxidase and measure the catalytic activity using a colorimeter. Record the results and measurements in a table, as shown below.
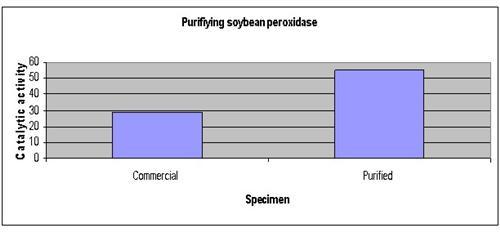
Results
The results show that the catalytic activity of the purified peroxidase was 60% higher compared to the unprocessed soybean peroxidase.
| Specimen | Commercial | Purified |
| Catalytic activity | 29 | 55 |
The graph below represents our results
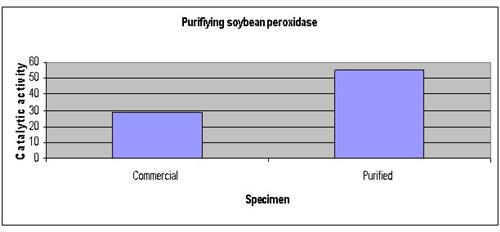
Conclusion
Our hypothesis that soybean peroxidase can be purified by liquid chromatography has been proven correct.
The discovery of soybean peroxidase is a very important step in bringing medical assistance to the needy in poorer nations. Using soybean peroxidase means that diagnostic kits for AIDS can be produced for the masses. The longer shelf life will also enable the medical kit to be stocked in more remote areas.
Also consider
The science fair project can also be done using different enzymes, like horseradish peroxidase.
Try to repeat the measurement at different pH solutions.
The experiment may be repeated using different concentrations of salt.
References
Better peroxidase improves disease diagnosis - http://www.sciencedaily.com/releases/1997/07/970715053558.htm
Soybean peroxidase – purification and some properties - http://ddr.nal.usda.gov/bitstream/10113/26349/1/IND81115894.pdf

