| Complexity level: | 9 |
| Project cost ($): | 50 |
| Time required: | 1 hour to prepare, 5 hours for the science project experiment |
| Material availability: | Easily found |
| Safety concerns: |
Hypothesis
Sand is able to keep water warm for the longest period time, when compared to air and Styrofoam.
Overview
Thermal insulation
Thermal insulators are materials that are used to reduce, minimize or prevent heat transfer from occurring. Heat transfer happens through conduction, radiation or convection. By covering the surface of a container with a layer or several layers of insulating material, heat can be prevented from either entering or leaving the container.
Generally, materials that are poor electrical conductors have been found to also be poor heat conductors. Electrical insulators like wood or plastic are also good thermal insulators. Good conductors like copper and aluminum are poor thermal insulators. Metals like copper contain many free flowing electrons on their surface that allow the transfer of thermal energy from one point to another.
Thermal insulation is also affected by the density of materials. A material with low density will act as a good thermal insulator. At a sub-atomic level, this can be explained because materials with low density will have atoms located further apart from one another. Therefore the transfer of energy from one atom to another will happen more slowly and less effectively. This is the reason why air is able to insulate heat better than water, and water is a better heat insulator than solids.
Scientific Terms
Materials
The materials required for this science project::
- 3 conical flasks
- 3 corks
- 1 electric drill with bits of the right size
- 3 thermometers
- 3 wooden boxes
- 1 bag of sand
- 1 bag of styrofoam balls or fibre
- Tap water
- 1 electric kettle
- 1 clock
MEL Physics — monthly physics experiment kits delivered to your door. (Affiliate link)
See what’s includedProcedure
1. For this experiment, the independent variable is the type of thermal insulator used – air, sand or styrofoam. The dependent variable is the temperature of the water in the conical flask. This is determined by using the thermometer to measure the temperature. The constants (control variables) are the size of the conical flask, the amount of water in the flask and the size of the wooden box.
2. The electric drill is used to make a hole in the center of the 3 corks. The size of the hole should be just slightly larger than the diameter of the thermometer. The thermometers are then inserted through the hole in the cork.
3. An electric kettle is filled with tap water and brought to the boil. Once the water has started to boil, it is poured into the 3 conical flasks. The corks with the inserted thermometers are used to close the opening on the top of the conical flask. The height of the thermometer is adjusted so that the bottom tip of the thermometer is immersed in the boiled water.
4. The first conical flask is placed inside an empty wooden box. The second conical flask is placed inside another wooden box and it is filled with styrofoam, up to the top of the flask. The 3rd conical flask is placed in the last box and filled with sand until the top of the flask.
5. The time on the clock is noted and the first temperature reading is taken from each of the three thermometers and recorded in the table given below. The temperatures are read from the 3 thermometers every hour for the next 5 hours and recorded in the table given below.
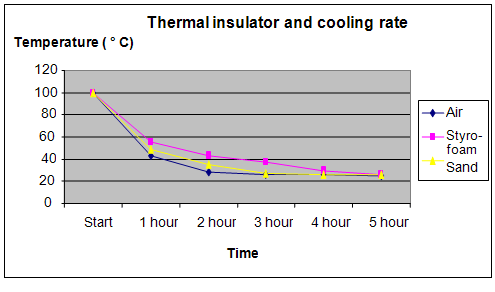
Results
It was observed that the water in the conical flask remained warm for the longest period of time, in the box filled with the styrofoam, followed by the box filled with sand.
|
Thermal insulator |
Temperature of the water in (°C) |
|||||
|
Start |
1 hour |
2 hour |
3 hour |
4 hour |
5 hour |
|
|
Air |
100.0 |
43.5 |
28.0 |
25.5 |
25.5 |
25.0 |
|
Styrofoam |
100.0 |
55.0 |
43.5 |
37.5 |
30.0 |
26.5 |
|
Sand |
100.0 |
48.5 |
35.0 |
27.5 |
26.0 |
25.5 |
The graph below represents the results of our science project experiment:
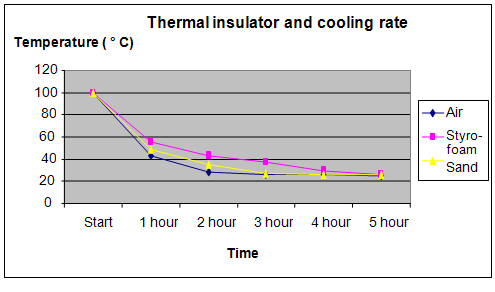
Conclusion
The hypothesis that sand is able to keep water warm for the longest period of time, compared to air and styrofoam is proven not to be true. Styrofoam actually kept water warm for the longest period of time.
Thermal insulation materials are used to prevent hot or cold objects from losing or gaining heat and having the same temperature as the surrounding environment. The thick sweaters that we use on cold days help keep our bodies warm. The insulating walls in our houses help keep the cool air inside on hot days. The thermos bottle is useful in keeping our drinks and food warm for a longer period.
Also consider
Try to repeat the experiment using ice cubes instead of boiling water.
The experiment can also be repeated using other insulation materials like cloth, newspaper or saw dust.
References
Thermal insulation - http://en.wikipedia.org/wiki/Thermal_insulation
Thermal insulation prevents hat from escaping - http://www.school-for-champions.com/science/thermal_insulation.htm

