| Complexity level: | 6 |
| Project cost ($): | 10 |
| Time required: | 1 day for preparation, 5 hours for experiment |
| Material availability: | Easily found |
| Safety concerns: |
Hypothesis
The freezing point of water will be lowered when sugar or salt is added.
Overview
Molar mass
The mass of 1 mole of an element (Molar mass) refers to 6.0221415 x 1023 atoms or molecules of the element. Although the SI unit of mass is in kilograms, molar mass is almost always mentioned in grams per mole. Salt has a molar mass of 5.8g per mol, while sugar has 34g per mol.
Freezing Point Depression
The term "freezing point depression" refers to a condition where the freezing point of a liquid is lowered by the presence of an additive. This is observed in all solutions, and the freezing point is determined by the amount of solute particles dissolved in the solvent.
At the freezing point of that particular solution, the chemical potential of the solution at liquid state and solid state will be equal. Solute particles are able to dissolve only in a liquid solvent. Conversely, when the solvent is in a solid state, solute particles cannot be dissolved. Following the same logic, adding a solute to the solvent will reduce the chemical potential of the solution and consequently, also its freezing point.
Scientific Terms
Materials
The materials required for this science fair project:
- 1 packet of sugar
- 1 packet of salt
- 800ml of distilled water
- 8 test tubes
- 8 beakers
- 1 basin
- Tap water
- 1 freezer
- Ice cube trays
- 8 thermometers
- 1 digital weighing scale
- 1 black marker
MEL Chemistry — hands-on chemistry experiment kits delivered monthly — great for building lab skills at home. (Affiliate link)
See what’s includedProcedure
1. For this science fair project, the independent variable is the amount of salt and sugar added to the solution. The dependent variable is the freezing point of the solution, and will be measured with a thermometer. The constants (control variables) are the temperature of the environment (which will remain at room temperature), the amount of solution in each test tube and the temperature of the ice in the basin.
2. Mix 200g of salt into in 1 liter of water and pour the solution into ice cube trays. Place the trays in a freezer, and leave overnight. The ice will be used to form an ice bath in the basin.
3. On the 2nd day, label the 8 beakers and test tubes as Salt 0.5M, Salt 1.0M, Salt 1.5M, Salt 2.0M, Sugar 0.5M, Sugar 1.0M, Sugar 1.5M and Sugar 2.0M.
4. Prepare 8 varieties of solution in each beaker as described below:
a. Salt 0.5M – Mix 100ml of water with 2.9g of salt
b. Salt 1.0M – Mix 100ml of water with 5.8g of salt
c. Salt 1.5M – Mix 100ml of water with 8.7g of salt
d. Salt 2.0M – Mix 100ml of water with 11.6g of salt
e. Sugar 0.5M – Mix 100ml of water with 17g of salt
f. Sugar 1.0M – Mix 100ml of water with 34g of salt
g. Sugar 1.5M – Mix 100ml of water with 51g of salt
h. Sugar 2.0M – Mix 100ml of water with 68g of salt
5. Fill a basin with the ice cubes prepared earlier, to create an ice bath.
6. Pour the solution in each beaker into its respective test tube to the halfway mark, or about a height of 5 cm. Place a thermometer into each test tube. Then, place the 8 test tubes into the ice bath.
7. Observe when the first ice crystals start to form, and record the temperature in a table, as shown below.
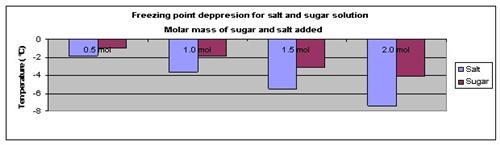
Results
The results show that adding more sugar or salt to the solution will lower its freezing point.
| Solute used | Freezing point of solution (°C) | |||
| l | 0.5 mol | 1.0 mol | 1.5 mol | 2.0 mol |
| Salt | -1.8 | -3.6 | -5.5 | -7.4 |
| Sugar | -1 | -1.9 | -3.1 | -4.1 |
The above results were then plotted onto a graph, as shown below.
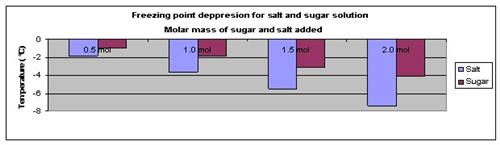
Conclusion
The hypothesis that a higher salt or sugar content in water will lower its freezing point has been proven to be true.
Water will normally freeze at 0 °C. However, seawater does not freeze at 0 °C, as the presence of salt lowers its freezing temperature to below 0 °C. The concept of freezing point depression is useful in applications where water needs to be kept liquid, especially in the use of antifreeze in combustion engines operating at freezing temperatures.
Also consider
This science fair project may also be repeated by comparing the effects of salt and sugar on the boiling point of water.
The experiment may also be repeated using a different solvent, like alcohol or thinner.
References
Freezing point depression - http://en.wikipedia.org/wiki/Freezing-point_depression
Molar mass - http://en.wikipedia.org/wiki/Molar_mass

