| Complexity level: | 8 |
| Project cost ($): | 20 |
| Time required: | 1 hour to prepare, 1 hour for the science project experiment |
| Material availability: | Access to basic laboratory equipment required |
| Safety concerns: |
Hypothesis
At temperatures above 40°C, the enzyme will become less effective.
Overview
The enzyme Catalase
Enzymes are proteins that help a certain chemical process take place. Chemical reactions require a certain amount of energy to take place. In the presence of the enzyme, the amount of energy required for the chemical process to take place is reduced. This happens because the enzymes bond with certain substrates to produce the end products. The reduction in the energy level helps to make the process happen faster.
Catalase is an enzyme which is found in almost all living organisms. This enzyme helps to convert hydrogen peroxide into oxygen and water.
Chemical activities that happen within the cell produces hydrogen peroxide, which is poisonous and can kill the organism. The presence of the enzyme catalase in the cell helps to quickly convert this toxic substrate into safer products of water and oxygen.
Scientific Terms
Materials
The materials required for this science fair project:
- 1 potato
- 1 blender
- 1 sheet of filter paper
- 8 beakers
- 8 test tubes
- 1 hot plate
- 1 packet of ice
- 1 bottle of hydrogen peroxide
- 1 stop watch
- 1 thermometer
- 1 pair of scissors
- 1 pair of tweezers
- 1 glass rod
- Marker pen
Procedure
1. For this experiment, the independent variable is the temperature of the potato and hydrogen peroxide. The dependent variable is the reaction time in the test tube. This is determined by measuring the time it takes for the paper to float to the top of the test tube. The constants (control variables) are the amount of hydrogen peroxide used, amount of potato used and the size of the filter paper.
2. The potato is cut into small pieces and, together with some water, blended until it becomes soft.
3. The filter paper is cut into 8 pieces of small squares 5mm by 5mm each, and each piece is then dipped in the blended potato using tweezers.
4. The 8 beakers and 8 test tubes are marked 1 to 8 using the marker pen. The test tubes are also marked with a line 8mm from the bottom of the tube. The hydrogen peroxide solution is poured into the 8 test tubes up to the level of the line.
5. The 8 beakers are filled with water up to 8cm high . By adding some ice to the water, the temperature of beaker 1 is brought to 15°C and beaker 2 to 20°C. Using the hot plate, the temperatures of the water in beakers 3 to 8 are brought to 25°C, 30°C, 35°C, 40°C, 45°C and 50°C. Place the test tubes marked 1 to 8 into the beakers marked 1 to 8 accordingly for 5 minutes, to enable the hydrogen peroxide to stabilize at the same temperature.
6. Put the filter paper dipped with the blended potato into the test tubes one at a time. Push the paper into the bottom of the test tube using the glass rod. Start the stopwatch and check the time it takes for the paper to reach the surface. Record the measurements in the table below.
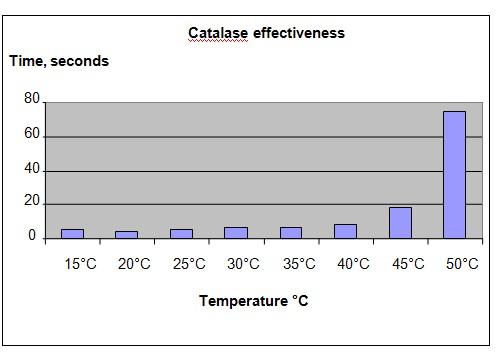
Results
It was observed that the filter paper floated very soon, at temperatures below 40°C. At temperature above 45°C, the paper took a longer time before floating.
|
Test tube number |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
Temperature |
15°C |
20°C |
25°C |
30°C |
35°C |
40°C |
45°C |
50°C |
|
Time taken for the paper to reach surface (seconds) |
5 |
4 |
5 |
6 |
6 |
9 |
18 |
75 |
The chart below represents the results of our experiment.
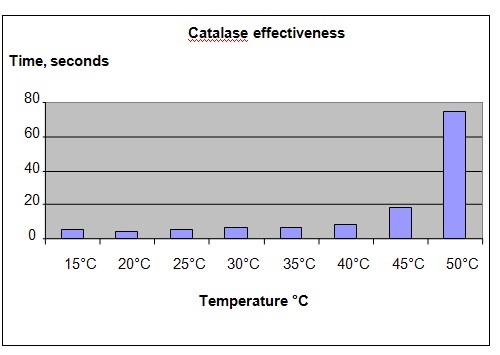
Conclusion
The hypothesis that at temperatures above 40°C, the enzyme catalase will become less effective, is proven to be true. Enzymes are made of proteins which will become unstable at higher temperatures. As the shape and structure of the catalase enzyme changes, its effectiveness as an enzyme is reduced.
Besides playing an important role in the preservation of life in living organisms, the catalase enzyme also has commercial uses. In the food industry, it is used to milk free from hydrogen peroxide content, so that the milk can be used to make cheese. In the textile industry, it is used to remove hydrogen peroxide from cloth.
Also consider
What will happen if the experiment is repeated using different amounts of hydrogen peroxide in the test tubes?
The experiment can be repeated using liver tissue instead of potatos.
References
Enzyme - http://en.wikipedia.org/wiki/Enzyme
Catalase - http://en.wikipedia.org/wiki/Catalase